In 2021, I wrote a blog post Helium vs. Hydrogen atom size exploring why I prefer hydrogen over helium for lighter-than-air applications. One of my key arguments was that hydrogen molecules, being larger (kinetic diameter 289 pm vs. helium’s 260 pm), should leak more slowly through the airship envelope. I likened this to a tea strainer-larger particles can’t escape as easily through small holes.
But I was recently contacted by Mr. Peter Garwood, historian and photojournalist of the Balloon Barrage Reunion Club, who generously shared his article on gas permeability during the barrage balloon era. His insights, backed by both history and physics, prompted me to revisit and correct some of my original assumptions.
The Misconception: Bigger Molecule = Less Leak
My original logic hinged on kinetic diameter alone, assuming that a bigger molecule like hydrogen would have more difficulty passing through small imperfections in fabric.
However, this overlooks the mechanism that actually governs gas leakage in airship envelopes: Effusion, not just Diffusion.
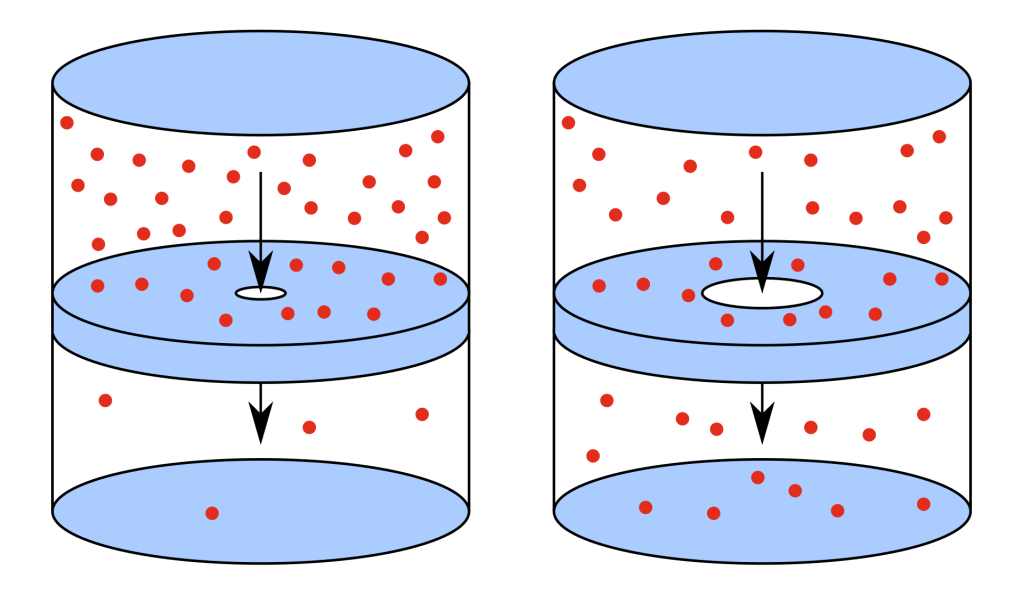
The Reality: Effusion Favors Hydrogen
Peter points to the Thomas Graham’s Law of Effusion, dating back to 1832, which says that lighter gases effuse faster, regardless of their physical size. In fact, under identical conditions, hydrogen effuses 1.4 times faster than helium, even though it’s “bigger”Gas Permeability.
“In simple terms this means that the larger Hydrogen gas is the ‘Houdini’ of gases and will leak faster than the smaller Helium gas.” – Peter GarwoodGas Permeability
That’s quite a turnaround! My earlier assumption neglected the role of molecular mass and internal pressure, which drive effusion. The “tea strainer” analogy doesn’t quite hold up when we’re dealing with quantum-scale particles under pressure.

Practical Implications for Airships
Well, despite hydrogen’s faster leak rate, I think it still holds major advantages for airship use:
- Hydrogen is ~6% lighter than helium, giving better lift.
- It’s renewable, whereas helium is finite and expensive.
- Envelope materials and temperature control can mitigate effusion to practical levels.
As I noted in the 2024 addendum to my original post, temperature also plays a crucial role—higher temperatures mean higher molecular activity and increased leakage. So while hydrogen leaks faster than helium at a molecular level, real-world airship performance depends on a broader set of factors: pressure, envelope materials, operational temperatures, and refuelling logistics.
Acknowledgment and Thanks
I want to thank Peter Garwood for getting in touch and providing his correction. It’s great that someone with his life-time experience invests his time into our little project.
If you’re interested in more of Peter’s work, check out the Balloon Barrage Reunion Club at www.bbrclub.org. It’s a treasure trove of historical knowledge from the era when these gases weren’t just theoretical.

One thought on “Effusion – “Helium vs. Hydrogen Atom Size” Review”